This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 Human biology
Human biology
From the very earliest times disease has struck fear into people. Even in the 21st century, new diseases can arise and cause havoc, as recent outbreaks of bird flu, H1N1 swine flu, Ebola and Zika virus show only too clearly.
The ability to find and manufacture a medicine which has the right effect in the right place is of vital importance in the fight against disease. Think of the impact that antibiotics have had on infectious diseases – illnesses that were life threatening 70 years ago are treated easily with a course of antibiotics today. And it isn’t just infectious diseases which need solutions. In the developed world, non-communicable problems such as heart disease, many different forms of cancer and type 2 diabetes affect the lives, and cause the deaths, of millions of people. Genetic diseases such as cystic fibrosis and sickle cell disease affect huge numbers of people. And the sequencing of the human genome is revealing that many non-communicable conditions have a genetic element to them – we are born with raised or lowered risks for many common conditions, depending on our genetic inheritance.
The pharmaceutical industry has responded to the work of the Human Genome Project with great enthusiasm. Detailed knowledge of the human genome is already making a number of potentially exciting advances possible in different areas of medicine development. The application of genomics is expected to produce more powerful medicines. This is because medicines can be designed to target specific diseases, or to respond to changes in the proteins or genetic material of the cells. Knowing the human genome it will be easier to produce medicines which affect pathogens or cancer cells but which do not damage healthy body cells. New ways of diagnosing diseases when they are relatively easy to treat becomes possible if we can read the genome of each person. In addition, in the future it is hoped medicines could be tailor-made to suit each of us as individuals.
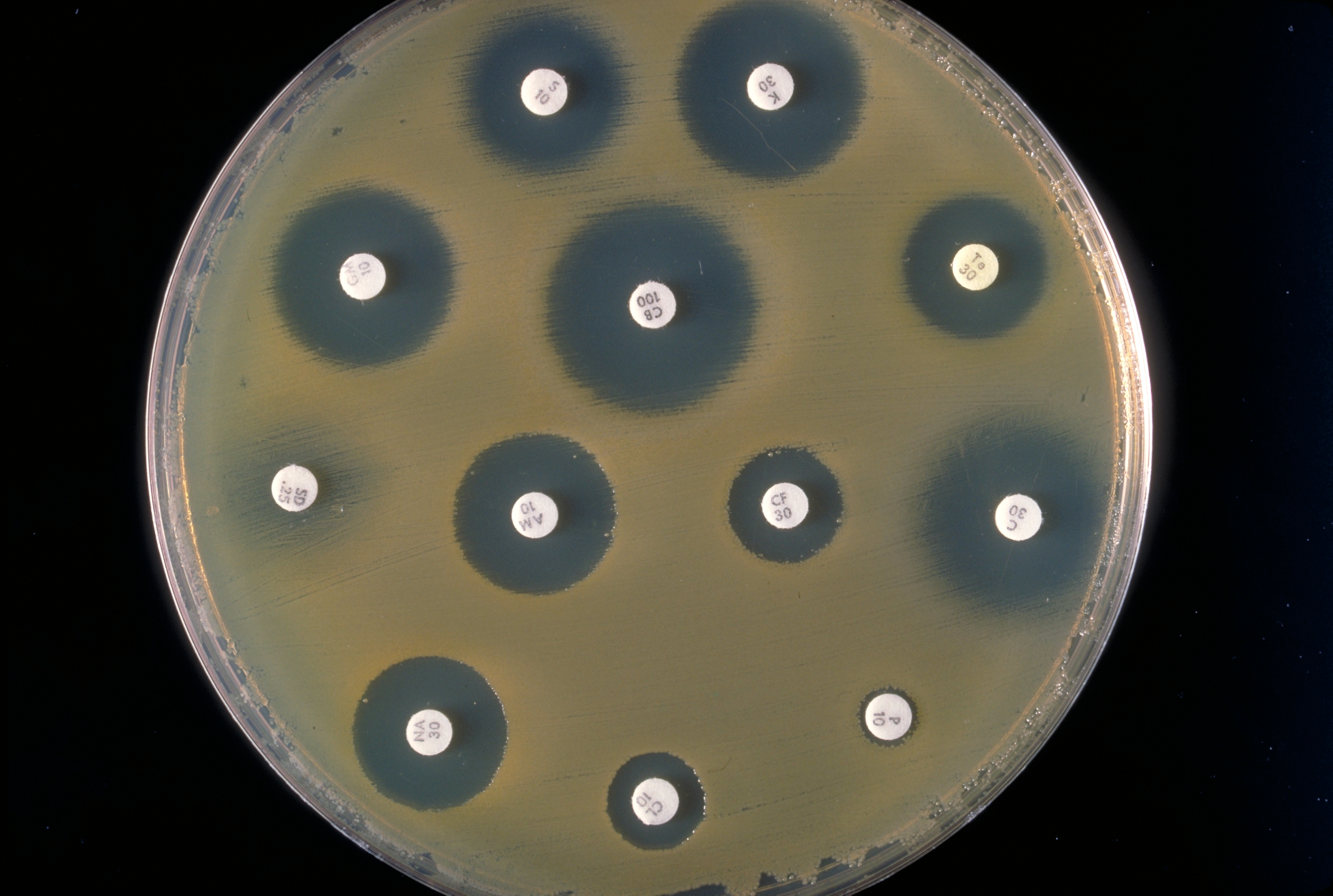
It isn’t just about sequencing the human genome. If the genome of a pathogen can be sequenced rapidly in a GP’s surgery, for example, antibiotics will never be prescribed for viral illnesses – and the most effective antibiotic can be prescribed against each pathogen. This would not only make cures more effective, it would also reduce the risk of antibiotic resistance developing in bacterial populations. The first disease for which scientists and doctors developed a diagnostic test based on genome sequencing was TB, in 2017. Sequencing the genomes of pathogens from patients with suspected TB enabled doctors to give a positive or negative TB diagnosis within days instead of the usual weeks or even months. What’s more, the most effective antibiotic could be used for treatment immediately. This means patients can be treated rapidly and effectively, the spread of the disease is significantly reduced and the risk of antibiotic resistance developing in the pathogens minimised. More and more diagnostic tests of this kind are ex
If medicines can be designed to work with our individual genetic makeup then they should work more effectively, in lower doses, with fewer side effects. The new science of pharmacogenomics has been developed to put together pharmaceutical expertise with the new knowledge of the human genome.
Research has already shown that genetic factors have a marked effect on the effectiveness of certain medicines. In some cases, the presence or absence of a particular enzyme which breaks down the drug molecule can have a major impact on the efficacy of the medicine. The presence or absence of suitable receptors can also make a difference. For example, medicines called kappa opioids appear to be much more effective pain killers in women than in men; it seems that women have many more receptors for the pain-killers, making them work more effectively.
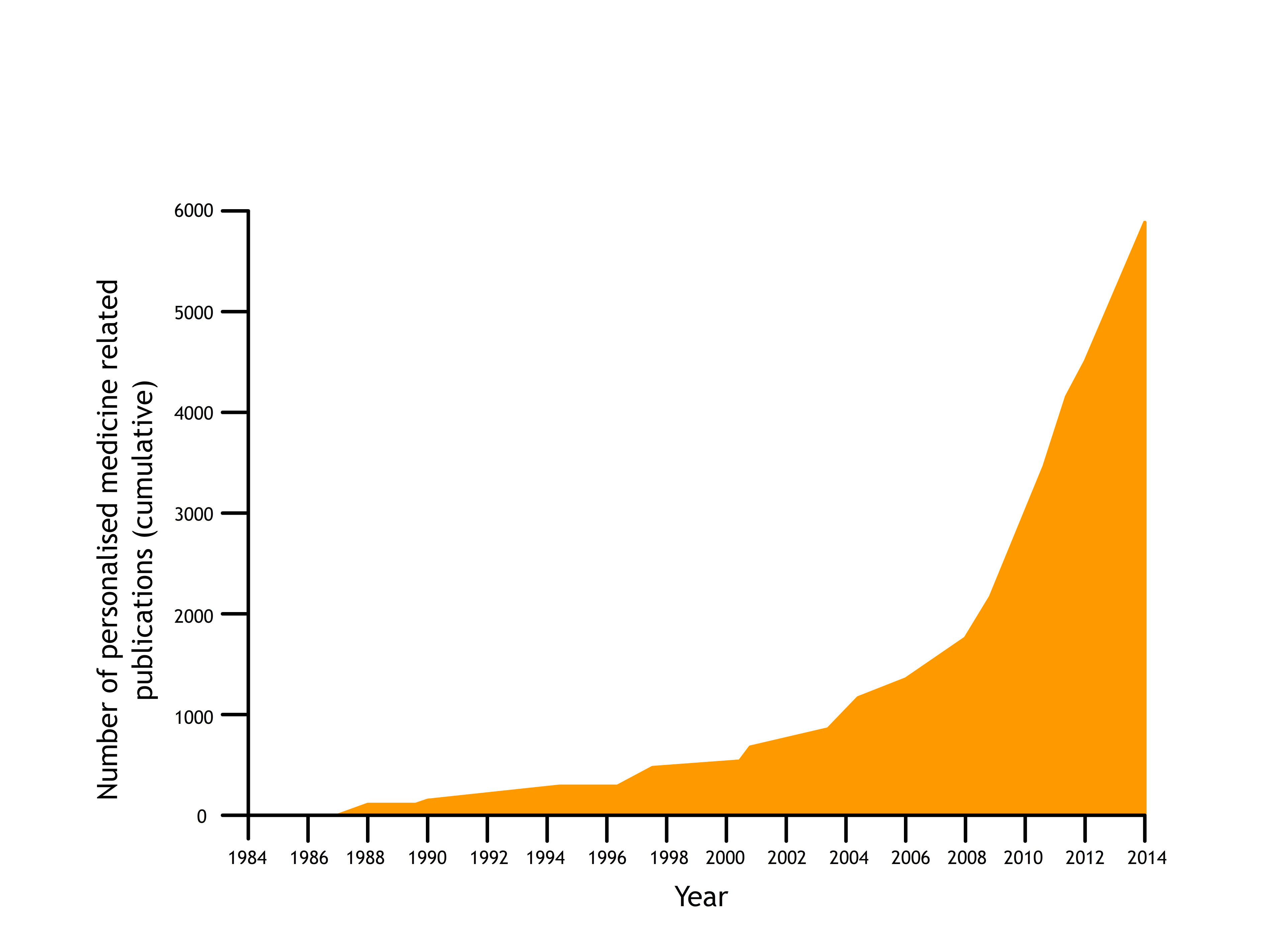
More detailed knowledge of our individual genetics is becoming available all the time from the findings of the Human Genome Project, the UK10K Project, the 100,000 Genomes Project and more. Our growing knowledge of the genome is already having an impact on the development of medicines, and scientists are working on algorithms to predict the people who are most likely to be helped by particular treatments.
But what are the current and possible future benefits of pharmacogenomics?
Understanding the human genome should make it much easier to prescribe the right medicine for a patient straight away. It is estimated that in the US around 100,000 people die each year, and two million people are hospitalised, as a result of being given a medicine which doesn’t suit them. If doctors can have access to a patient’s genome they will be able to prescribe medication which will work with rather than against their DNA. This approach results in personalised medicine – also known as stratified medicine - with treatments tailored to each individual and their needs. For example: a medicine known as abacavir is used, in combination with other retrovirals, in the treatment of HIV infections. It is very effective at helping delay the onset of full-blown AIDS for many years. Unfortunately, 5-7% of the patients treated with this drug are hypersensitive to it. They have a very powerful, allergic response and may even die as a result. Scientists have pinpointed the genetic variant which leads to this response, and patients are screened before they are treated with abacavir. As a result, serious side effects are now very rare indeed – and the patients who would react to the drug can be offered an alternative treatment which is safe for them.
Another important improvement will be in the dosages of medicines used. Taking the age or weight of a person and using it to decide what dose of a medicine they should be given is a fairly random way of doing things. Genetic information about individuals will allow doctors to work out just how rapidly each person deals with a particular medicine and excretes it from their body. Many people could have much lower doses of medicines, whilst those who need it could be given higher doses.
For example:
Codeine is a drug which undergoes biotransformation to become active as a pain killer. Some genetic variants mean people produce little of the enzyme needed to transform codeine into its active form, so the drug has little pain-killing effect. They need a much higher dose or a different painkiller. Others are ‘super-transformers’ – they transform codeine to its active form very effectively and so they can be given a much lower dose to have the same painkilling effect.
Cystic fibrosis is a genetic disease caused by one of a number of mutations affecting the cystic fibrosis transmembrane conductance regulator (CTFR) transport protein in the cell membrane. A new drug has been developed which directly targets the defective transport system in one variant of cystic fibrosis, giving huge benefits in terms of quality of life. Genome sequencing makes it possible to identify the patients who will benefit from this treatment, without raising the hopes of those it will not help.
Personalised medicine isn’t just about medicines and treatments. Unravelling the genome is making many new, accurate diagnostic tests available to health professionals. Examples include:
Identifying individuals with specific variants of the BRCA1 or BRCA2 gene who have a greatly increased lifetime risk of developing breast or ovarian cancer as a result of their genetic combination. They can be closely monitored and offered treatments to reduce their risk.
Some families have a genetic tendency to very high cholesterol levels, which in turn can lead to cardiovascular disease. The high cholesterol is not easily lowered by diet or exercise – it requires medication. It is only picked up in about 15% of the population using normal GP screening. Information from the genome enables people at risk to be identified and treated before their arteries or hearts suffer damage.
Advances like these are of enormous benefit to patients, and they will also save the NHS a great deal of money in a number of ways:
by diagnosing people correctly, and in the earliest, treatable stages of diseases such as cancer
by reducing medicine wastage, only treating people who will benefit from a particular drug
by avoiding the expense to the NHS, and distress to individuals affected, when people either are not helped by a medicine or react badly to the medicine they have been given.
Personalised medicine, based on increasingly rapid, cheap genome sequencing, holds out great hopes for the future of effective medicine, both in the UK and around the world.