This topic takes on average 60 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 Chemistry
Chemistry
 Physics
Physics
 Science
Science
The three different states are solid, liquid and gas.
These all look and feel different, but also have different particle arrangements and energies.
Particles have bonds between them which can be formed or broken, changing the state of the material. Such disruptions to bonds can happen due to changes in energy. For example, heating a solid (such as ice) increases the energy of particles and allows the bonds between them to break. This means that the particles spread out, and the solid ice becomes liquid water.
Changes of states are reversible, meaning that if a solid is melted into a liquid by heating, it can be changed back to a solid if cooled again (as an example).
Watch the videos below which explain changing states of water and reversible and irreversible changes. Think about how this could relate to the water cycle.

Reversible and non-reversible changes.
Some changes are reversible.
Once you have changed something you can change
it back.
Like melting ice to liquid then refreezing
it again.
Some changes are non-reversible.
Once you have changed something you can not
change it back, like cooking an egg.
Or mixing flour and milk.
Or burning a match.
Changing states of water.
When the water is boiling we see bubbles but these are not bubbles of air they are bubbles of water that have changed state to a gas.
This happens at approximately 100 degrees celsius.
You need to give the water energy, heat, to turn it into a gas.
If you change the amount of heat it can change how fast the water changes state into a gas.
The diagram below shows the particle arrangements and reversible processes in changing states.
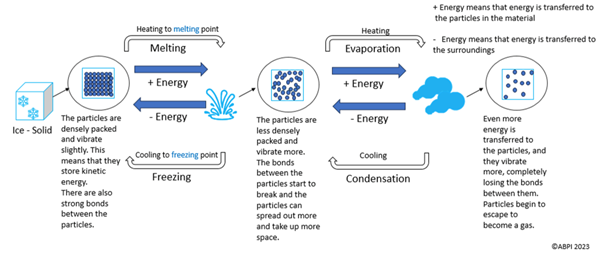
One process that is not highlighted in the diagram is sublimation. This is when a solid is converted directly into a gas, missing the liquid stage. An example is dry ice. Like the above processes, sublimation is also reversible, and the reverse process is known as deposition.