This topic takes on average 90 minutes to read.
There are a number of interactive features in this resource:
 Chemistry
Chemistry
 Physics
Physics
Carbon is recycled through the carbon cycle which includes both natural and human activities.
Natural cycling includes photosynthesis and respiration, whilst human carbon cycling involves the combustion of fossil fuels.
Burning fossil fuels is known as combustion as the carbon from the fuel combines with the oxygen in the air to release carbon dioxide (CO2) and water vapour (H2O).
For combustion to happen, fuel, heat and oxygen are all needed. If one of these things is removed, combustion will stop and the fire will go out.
The combustion of fossil fuels is also an oxidation reaction. An oxidation reaction is when an element combines with oxygen atoms to form a different molecule (in this case, carbon combines with oxygen to make carbon dioxide - CO2).
That is why, if oxygen is removed, the combustion will stop. During oxidation, electrons are lost. This is the opposite of a reduction reaction, where electrons are gained and oxygen is lost.
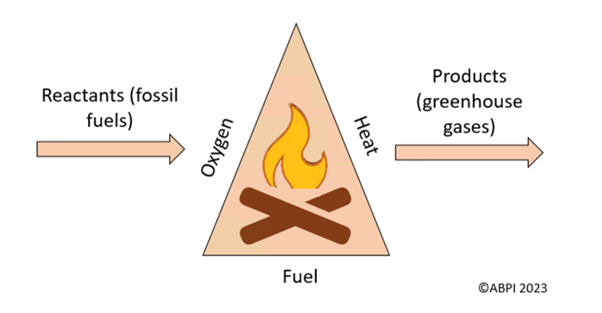
A good way to remember the difference between oxidation and reduction is OIL RIG:

The reactants of the combustion reaction contain carbon and oxygen elements.
The product of an oxidation reaction will always be an oxide (such as cardon dioxide – in this case ‘di’ as there are two oxygen atoms).
Below is an equation to show the combustion of butane.
Butane is a gas which is commonly used to generate household energy for cooking and heating. It is a fossil fuel, meaning that it contains carbon which can be made into carbon dioxide.
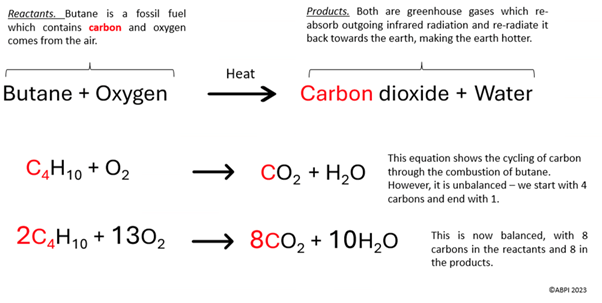
One of the products from the combustion of butane is CO2.
Carbon dioxide is a greenhouse gas and is chemically known as a compound. Compounds are made when two or more elements combine, and in this case, it is carbon and oxygen. These elements always combine in a fixed proportion, so, a carbon dioxide molecule is always one carbon and two oxygen atoms to make CO2.
This means that carbon is not the same as carbon dioxide.
For carbon to become carbon dioxide, combustion or respiration is required.
Respiration is a natural process which does not significantly contribute to global warming. Instead, it is the combustion of fossil fuels which contributes the most significantly. This is a non-renewable energy source.