This topic takes on average 45 minutes to read.
There are a number of interactive features in this resource:
 Chemistry
Chemistry
 Science
Science
Although greenhouse gases are pollutants, not all pollutants are greenhouse gases.
Greenhouse gases are the pollutants that can specifically absorb outgoing infrared radiation and re-radiate it back towards the Earth.
However, not all pollutants can do this. For example, particulate matter is a pollutant but not a greenhouse gas. Instead, these particles can form soot.
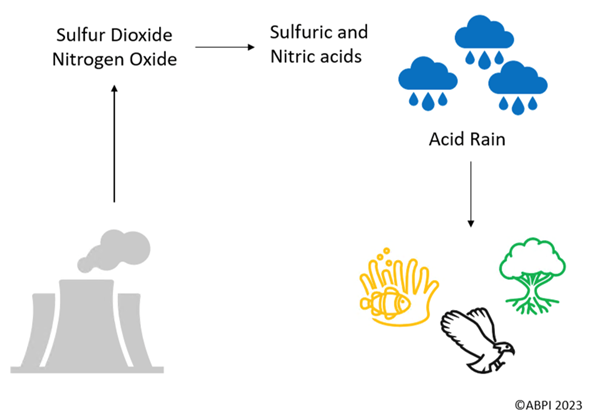
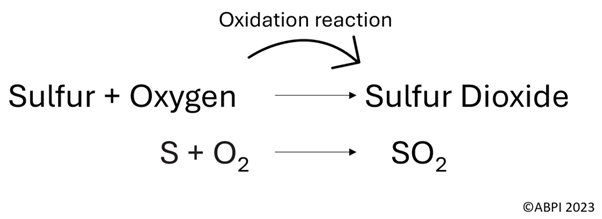
Sulfur dioxide (SO2) is a pollutant which is formed when a fossil fuel that contains sulfur is combusted.
An oxidation reaction occurs, and the sulfur in the fossil fuel combines with oxygen to form sulfur dioxide. An oxidation reaction is when an element combines with oxygen atoms to form a different molecule.
The release of sulfur dioxide as a pollutant harms the environment, animals and humans.
Sulfur dioxide can also go through more reactions to form sulfuric acid. Sulfuric acid is a component of acid rain, which harms the environment and can damage buildings.
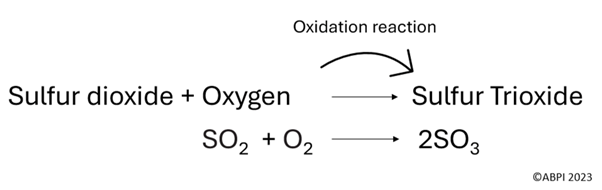
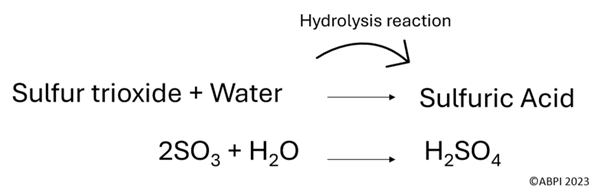
Note that during oxidation reactions, an electron is lost but oxygen is gained. Also note that the above equations are not balanced.
Nitrogen oxides are also pollutants. Though fossil fuels do not contain nitrogen themselves, the air does, and so when fossil fuels are combusted, this causes oxidation of nitrogen too. This forms both NO and NO2, which have harmful effects on human health. Nitrogen oxides can contribute to acid rain too, in the form of nitric acids.
The consequence of this is that the ocean is becoming too acidic for aquatic life, which also affects the food chain. For example, this affects birds who eat fish, as they have reduced food availability. In fact, the pH of the ocean is around 8.1 but it used to be around 8.2. Though this seems like a small change, it is big enough of a difference for aquatic life to be affected.
Acidic oceans also place threat on coral reefs.
Acid rain can be absorbed by the ground too, removing nutrients from the soil which plants and trees need to grow.