This topic takes on average 90 minutes to read.
There are a number of interactive features in this resource:
 Chemistry
Chemistry
 Physics
Physics
 Science
Science
Alkenes are unsaturated hydrocarbon chains with at least one double bond. Just like the alkanes, it is important to know the first four in the series. These are ethene, propene, butene and pentene.
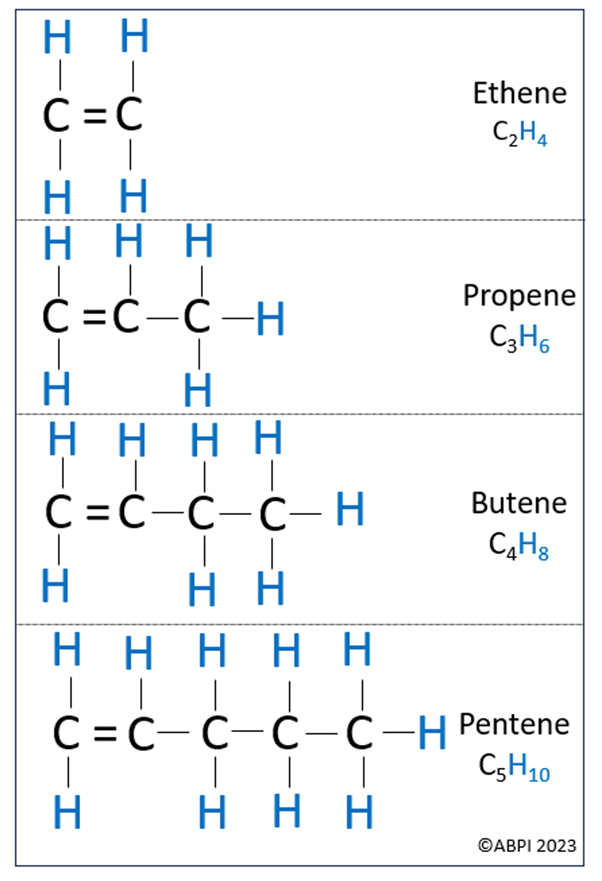
When drawing the alkenes, remember that each carbon can only have 4 bonds – therefore, if a carbon has a double bond, it must lose one of the hydrogen bonds.
Plastic is a very common material used today, and this is a polymer.
Polymers are solid at room temperature since their intermolecular forces are strong.
During addition polymerization, polymers are made up of lots of alkenes, such as ethene. The alkene (ethene) is known as a monomer.
Ethene has a double carbon to carbon covalent bond (represented as = ).
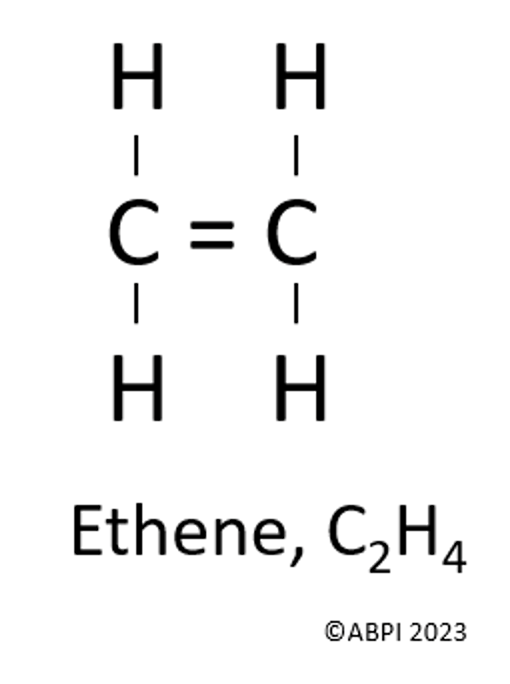
Poly(ethene) is the most common plastic. As the name suggests, this is made by joining lots of ethene monomers together (as poly means many).
In the polymer the double bonds have been broken and there are single carbon to carbon covalent bonds instead. The reason that alkene monomers can form polymers is because of their double bond; the double bond can open, allowing two monomers to bond together.
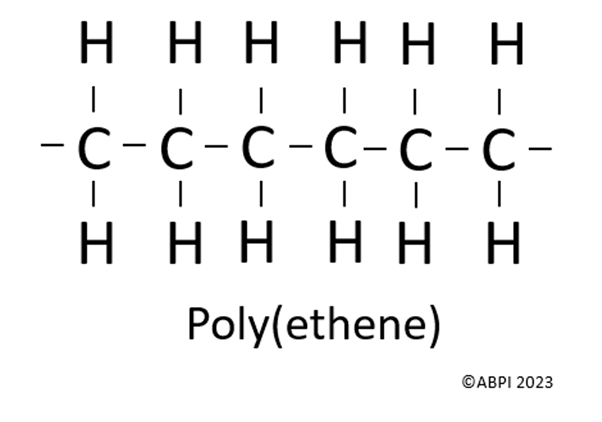
Many more single ethene units bond together to form polymers, but this can be hard to draw out. Therefore, scientists often use this shorthand repeated unit formula instead:
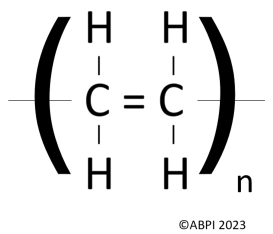
Whereby n = a very large number of repeating units. A number can be given to display exactly how many.