This topic takes on average 90 minutes to read.
There are a number of interactive features in this resource:
 Chemistry
Chemistry
 Physics
Physics
 Science
Science
Butane is one of the last to exit the fractional distillation column due to its short hydrocarbon chain, and this is why it is so useful. The hydrocarbon chains which exit first are not very useful though as they are too long and not very flammable. Therefore, these longer chains can go through another process known as cracking to make them shorter and more useful.
Cracking can be performed using either catalytic cracking or steam cracking. Both are thermal decomposition reactions.
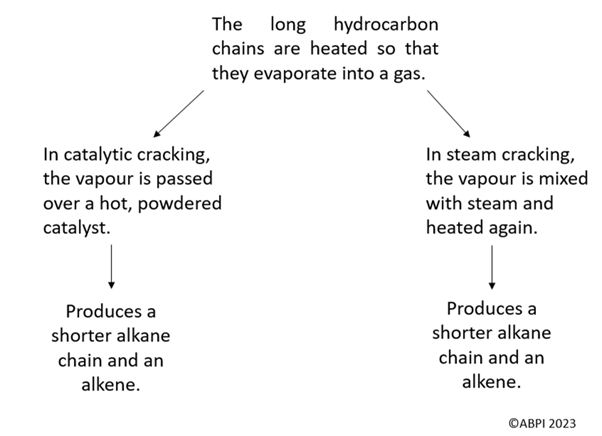
The process of cracking produces both a shorter alkane and an alkene.
Alkenes are similar to alkanes, but they have a carbon-to-carbon double bond in their hydrocarbon chain, meaning that they are unsaturated. They are also more reactive than alkanes, meaning that they can turn bromine water from orange to colourless. This is how scientists would test for the presence of an alkene.
We can describe the cracking process as a chemical equation, for example:
C6H14 → C4H10 + C2H4
Whereby the reactant (C6H14) is an alkane, and the outputs are an alkane and an alkene, respectively.
The general formula for an alkane is CnH2n+2, and the general formula for an alkene is CnH2n. This can be used to confirm whether the cracking equation is correct. The number of carbon and hydrogen atoms in both the reactants and products must also match for the equation to be correct.