This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 PSHE / Citizenship studies
PSHE / Citizenship studies
 Science (applied)
Science (applied)
Duchenne muscular dystrophy (DMD) is the most severe form of muscular dystrophy. It affects about one in every 3500 boys who are born – about 100 boys a year in the UK. It is a sex-linked genetic condition which means the boys cannot make a protein called dystrophin, a protein vitally important for maintaining healthy muscles. Without it the muscles weaken and waste away, being replaced by fat, so that by their early teens most affected boys are confined to a wheelchair and their life expectancy is only to early adulthood.
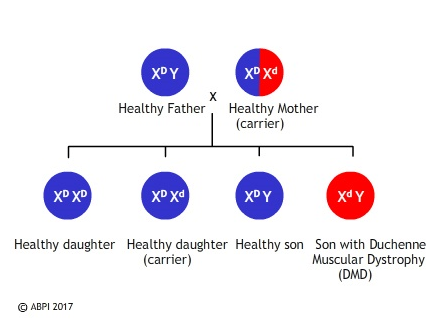
Duchenne muscular dystrophy is a sex linked recessive disease, inherited on the X chromosome
The faulty gene is very large, which makes normal gene therapy techniques difficult. However researchers in the United States and in Britain have found ways of using parts of a healthy gene, called mini-genes, to repair the damaged DNA, enabling the muscles to produce dystrophin and to function in a much more normal way. What is more, the effect has been long term – the protein was still being made a year after the gene was inserted. The only problem is that the gene therapy technique has so far only been tried in mice and golden retrievers, which have a natural mutation similar to muscular dystrophy.
Much of this research depends on knockout mice. To produce knockout mice researchers genetically modify some embryonic stem cells to inactivate or ‘knock out’ a healthy gene. These cells are then injected into mouse embryos which are then implanted into a surrogate mother. The mice which result have some knockout cells and some normal cells, and they are then implanted to produce homozygous knockout mice.
Knockout mice often show changes in their phenotype which mimic human genetic problems, helping scientists understand exactly what the gene does.
Knockout mice are also useful for studying the impact of different therapies. We have many of our genes in common – of 4000 genes studied in mice and humans, only ten of them are found in one species but not in the other. This, along with the fact that mice reproduce rapidly, have large litters, and are easy and cheap to keep means that knockout mice are incredibly useful in our search to understand gene functions and to find cures for many diseases.
The problem with the mdx mice (a popular model for studying DMD) is that they only display relatively mild symptoms. Several breeds of domestic dog have also been found to have a natural mutation in the dystrophin gene and some work has been done on golden retrievers. Dogs are not ideal laboratory animals for many reasons – they are intelligent and emotive, they are not easy to manipulate genetically, and they take time and effort to breed. However, dogs affected by the canine form of Duchenne muscular dystrophy do have symptoms which are very similar to humans. Now a team at the Royal Veterinary College have discovered a line of King Charles spaniels which appear to have the same mutation in the same gene as humans. A research project began in 2015 looking at the progression of the disease in this breed of dog. This may in future lead to improved therapies for humans and dogs alike.

Muscular dystrophy in King Charles spaniels is caused by a mutation in the same gene as Duchenne muscular dystrophy in humans, so treatment developed to help one species should also help the other.
Many of the current trials on possible treatments for DMD still involve the use of medicines to alleviate symptoms, but there have been some promising results recently with genetic modification in both mice and dogs. A few phase 1 human clinical trials are in progress and more are expected soon. Some scientists are attempting to replace small regions of the faulty gene, others are trying to replace the whole thing. Gene therapy has not yet been fully successful in overcoming any genetic diseases, so any patients who take part in early trials of a possible new treatment – and their parents – are very brave. New technologies such as CRISPR-Cas9 hold out hope for new therapies including editing muscle-forming stem cells rather than trying to change the whole organism. There is a long way to go, but muscular dystrophy is another disease where gene therapy may eventually result in a treatment or even a cure.
See:
Knockout Mice Fact Sheet, National Human Genome Research Institute
Why Mouse Matters, 2000 Mouse Sequencing Consortium, National Human Genome Research Institute
A new animal model of Duchenne muscular dystrophy, Muscular Dystrophy UK
Animals are frequently used in scientific research.
What are some arguments for and against this?
