This topic takes on average 75 minutes to read.
There are a number of interactive features in this resource:
 Chemistry
Chemistry
 Biology
Biology
Around 18% of the human body is made of protein – second only in mass to water. Proteins are important in living organisms in many ways including:
Like carbohydrates and lipids, proteins include the elements carbon, hydrogen and oxygen but proteins also all contain nitrogen. Many proteins also contain sulfur and some include phosphorus and other elements.
Proteins are both macromolecules and polymers – they are built up of long chains of monomers called amino acids.
Nine of these are essential amino acids – we must eat them in our diets as we cannot synthesise them in our bodies. Eleven of the amino acids are non-essential; although we usually get them from our food we can synthesise them if we need to.
All amino acids have the same basic structure with an amino group (-NH2) and a carboxyl group (-COOH) attached to the same carbon atom, along with a hydrogen atom and a variable group often referred to as the R group. The carboxyl end is acidic and the amino end is basic (i.e. has the properties of a base, can accept a proton H+ ion) in nature so amino acids are amphoteric – they can act as acids or bases. The R group – see below – also affects the nature of the amino acid and has a big effect on the structure of proteins.
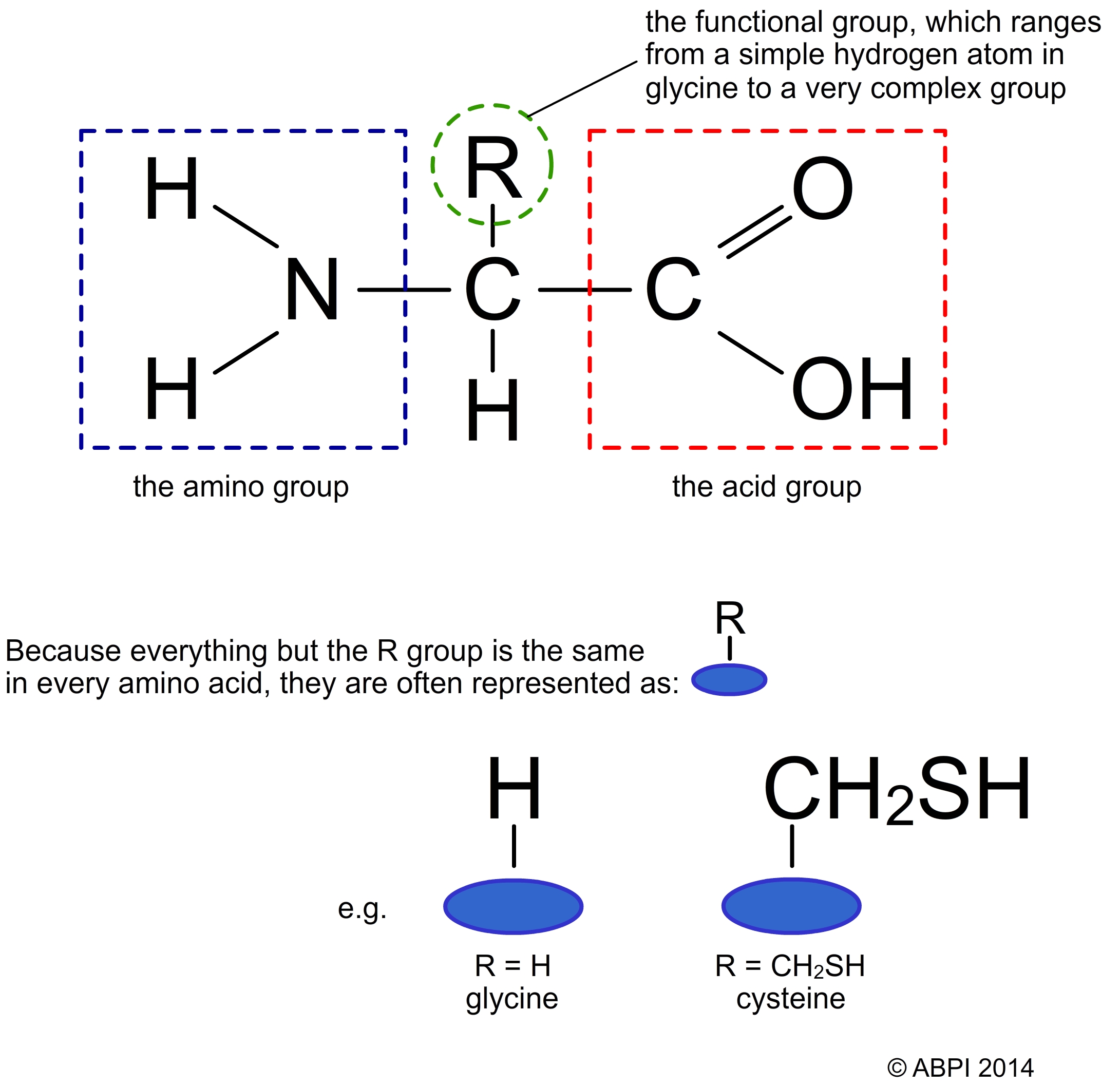
Structure of an amino acid
Although the R group is important in the structure of proteins, it is ignored when considering how amino acids join together to form proteins.
Amino acids are joined in a reaction between the amino group of one amino acid and the carboxyl group of another. This is a condensation reaction which results in a bond known as a peptide bond (or peptide link). Two amino acids joined form a dipeptide. As more and more amino acids are joined into the chain it becomes a polypeptide chain, which may contain hundreds or even thousands of amino acids. A protein is a polypeptide chain which is folded or coiled or associated with other polypeptide chains.
Peptide bonds are broken in a hydrolysis reaction to release the amino acids.
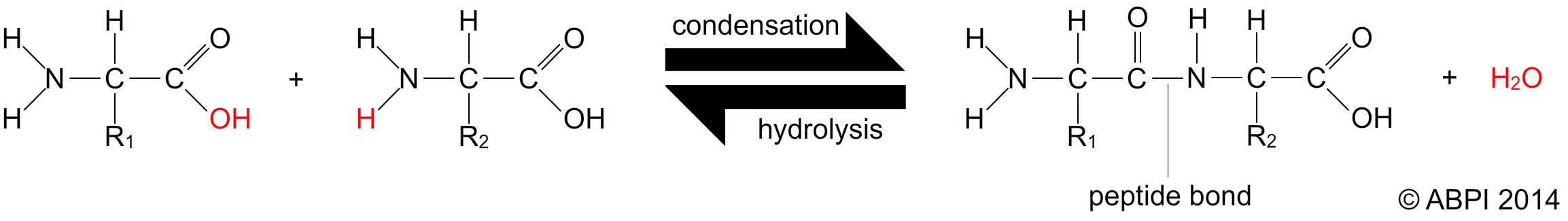
Amino acids are joined together to form polypeptides and proteins by peptide bonds.