This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 Human biology
Human biology
 PSHE / Citizenship studies
PSHE / Citizenship studies
 Science (applied)
Science (applied)
In 2003, the entire sequence of the human genome was published for the first time. This heralded the dawn of personalised medicine - the medicine of the future. The genome is all of the DNA found in your cells, including both the nuclear and the mitochondrial DNA.
Since the original Human Genome Project, scientists have analysed thousands of human genomes. The technology by which DNA samples are amplified and sequenced has moved forward in leaps and bounds since the early days of the project and continues to develop fast. Analysing an entire human genome, which took years and cost millions of pounds the first time it was completed, now takes a few days and costs thousands of pounds. Scientists expect that, with the current rate of technological development, the time will come when every hospital and even every GP surgery will have the technology to sequence the DNA of their patients - and the pathogens that attack them.
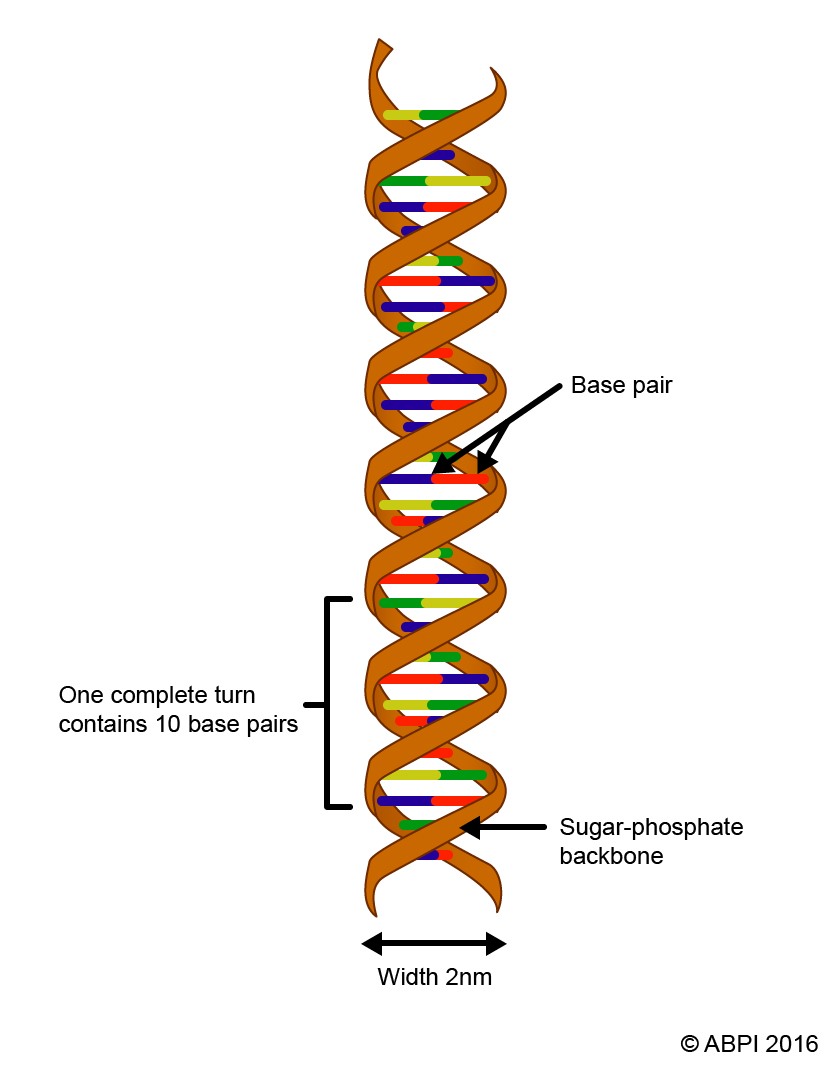
DNA holds the secrets of our health - and our risk of disease.
The concept of personalised medicine is growing and becoming more important all of the time. The essence of personalised medicine is that the health care and medical treatment of each individual will be uniquely matched to their needs, based largely on an analysis of their genetic information. This not only means that the health risks of each individual can be carefully measured and monitored, it also means that drugs can be developed and tailored to fit individual genotypes . This new direction for the pharmaceutical industry is often referred to as pharmacogenomics.
By analysing the genomes of many different people scientists are building up a picture of how our genome affects our health. This ever-developing knowledge base is being used in a number of ways already. These include:
For example, certain genetic combinations are now known to be associated with an increased risk of developing heart disease, or breast cancer. If individuals with a high risk genotype can be identified, they can make lifestyle choices that minimise their risk of disease. They can also be screened regularly for indications of the onset of these diseases.
Scientists at the Wellcome Trust Genome Campus are working with patients who develop cancer, sequencing the genome of the patient and their cancer and then sequencing the genome of the tumour as the disease progresses. They are finding that the cancer mutates and changes throughout the disease. This may be why in many cases chemotherapy is only effective for a certain length of time. By tracking the changes in the cancer genome, doctors may be able to make changes in the medications to target the 'new' cancer, increasing the success of treatment over the long term.

Genome sequencing is becoming faster and cheaper all the time
(Photo Credit: Shaury Nash)
Genome sequencing opens up the possibilities of developing drugs that are particularly effective for people with particular genotypes. Pharmacologists and doctors already know that different people react differently to the same drug. A drug that has dramatic benefits in one group of patients may have little or no effect in others and cause unpleasant side effects for some. The way the cells of your body respond to particular drugs, and the way drugs are metabolised and excreted by your body, are all determined by your genetic makeup.
The hope is that in the future scientists will be able to use the genome of an individual to identify which drugs will work best for them at the lowest possible dose. This has huge potential both for individuals who will get more effective treatment, for society, with healthier populations and fewer people adversely affected by medications, and for scientists developing the drugs who will have a greater chance of successful drug development if, from the outset, they can target the drug only at the group of the population who will get most benefit from it. You can find out more about pharmacogenomics at http://www.yourgenome.org/facts/what-is-pharmacogenomics.
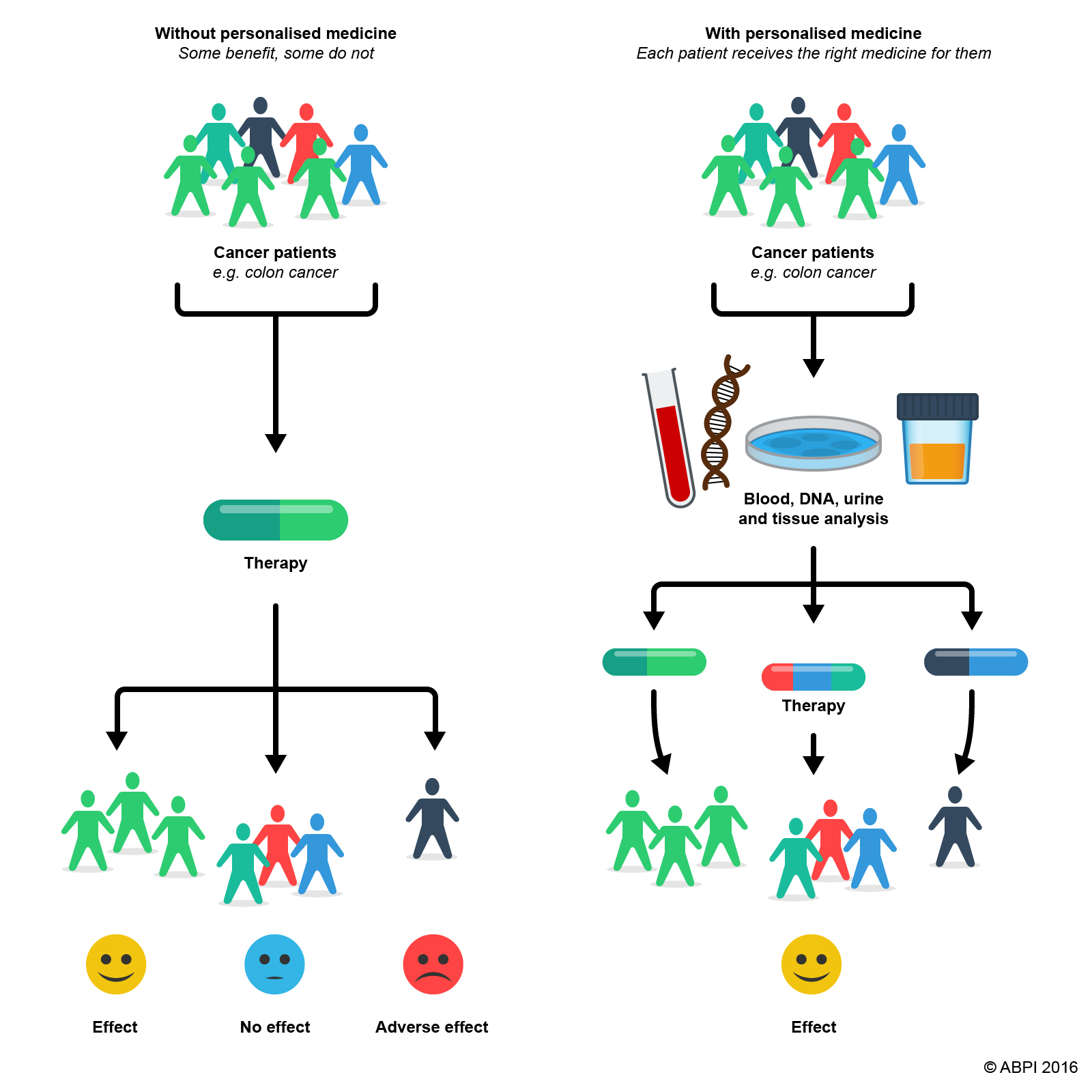
Pharmacogenomics can make personalised medicines a reality
The world of medicines is developing fast. Armed with an intimate knowledge of both the human genome and the genomes of our many pathogens, along with ever-increasing computer power, the pharmacists of the future will be able to develop an array of treatments that we cannot even imagine now.
When doctors have the genomes of all their patients available to them, they will be able to identify the people who are at risk of developing particular diseases and target them with protective medications, suggesting sensible lifestyle choices and treat conditions that do occur in a precisely targeted way.
Each individual will be treated with drugs that work with rather than against their own biology, and side effects will be reduced to a minimum. In theory at least, the effect on each of us as individuals will be enormous, and society as a whole will reap the economic benefits of a healthier population.