This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Science
Science
 Biology
Biology
Antimicrobials have had an enormous impact on the health of humans and animals. After their discovery and development in the 1920s-30s, use of these medicines grew dramatically. Over time, their use has changed in response to concerns over issues such as antimicrobial resistance and antimicrobial residues. Today, to treat infections in animals and humans, antimicrobials must be prescribed by a health worker (e.g. doctor or vet), and campaigns have been set up to ensure they are used appropriately. By ensuring that antimicrobials are only available on prescription, access to these important products can be controlled helping to prevent misuse. Doctors and vets play a key ‘gate-keeping’ role in controlling access in this manner.
Penicillin was discovered by Alexander Fleming in 1928 and refined by Howard Florey and Ernst Chain in 1941. Since this first discovery, several antimicrobials have been discovered. Concerns about antimicrobial resistance were raised early on in their use. In 1945, Fleming, Florey and Chain were awarded the Nobel prize for the discovery of penicillin. In Fleming’s acceptance speech he noted that, ‘it is not difficult to make microbes resistant to penicillin in the laboratory by exposing them to concentrations not sufficient to kill them, and this has occasionally happened in the body.’

The benefits of antimicrobials to food production saw their use grow significantly. Antimicrobials were used in applications that were not for treating or preventing disease. In 1949, it was reported that treating a healthy animal with some forms of antibiotic could promote the growth of animals, improve egg production in hens, increase the number of calves per cow, and increase milk yield in dairy cows. Antibiotics added to animal feed to increase the weight of animals were titled antibiotic growth promoters.

In 1958, it was estimated that up to 50% of pigs were administered antibiotics via their feed, and many piglets were administered antibiotics called tetracyclines via medicated feed formulations (a class of antibiotic that increased the weight of livestock, such as pigs)1. By the 1960s, antibiotic use was increasing in healthcare and throughout global food production1. Between 1951 and 1970, total antibiotic use in the US increased from 690 to 7,670 tons, with nonmedicinal use increasing over 30-fold from 110 to 3,310 tons1. With a growing global population and an increased demand for animal products in developing countries, farmers were under pressure to produce more animals more effectively, and antibiotics helped achieve this.
In the 1970s, the public become concerned about levels of antibiotic residues in the food that they could be exposed . As a result, many countries began programs to monitor the levels of antimicrobial residues in foodstuffs.
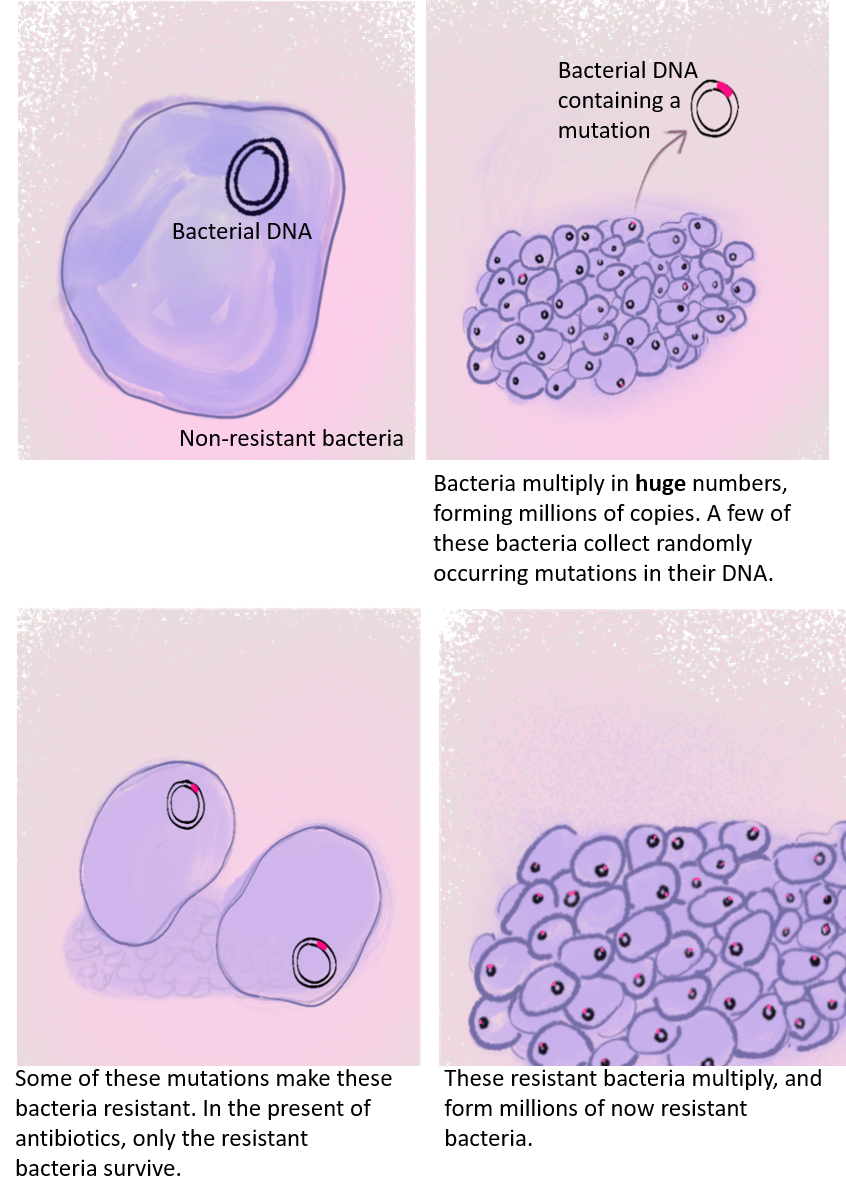
Researchers began to warn about the rising levels of antimicrobial resistance in the 1980s. The discovery of new antibiotics had started off well, but over time it became clear that searches for new antibiotics for humans and animals had stalled, and bacteria had begun to develop resistance to the commonly used antibiotics2. In Denmark, resistant pathogens were identified in healthy pigs and chickens in 1993, and the country phased out the use of antibiotic growth promoters. In 1994, 115,786kg of antibiotics were used in Denmark; in 1999 this had dropped to 12,283 kg. The EU and the UK stopped the sale of antibiotic growth promoters completely on 1st January 20063. The practice of using antibiotics for growth promotion in the UK is now entirely illegal. Farmers can gain access to antimicrobials only through vets to treat infections. Antibiotics only available with a veterinary prescription.
The US also bans the use of antibiotics for growth promoter purposes.
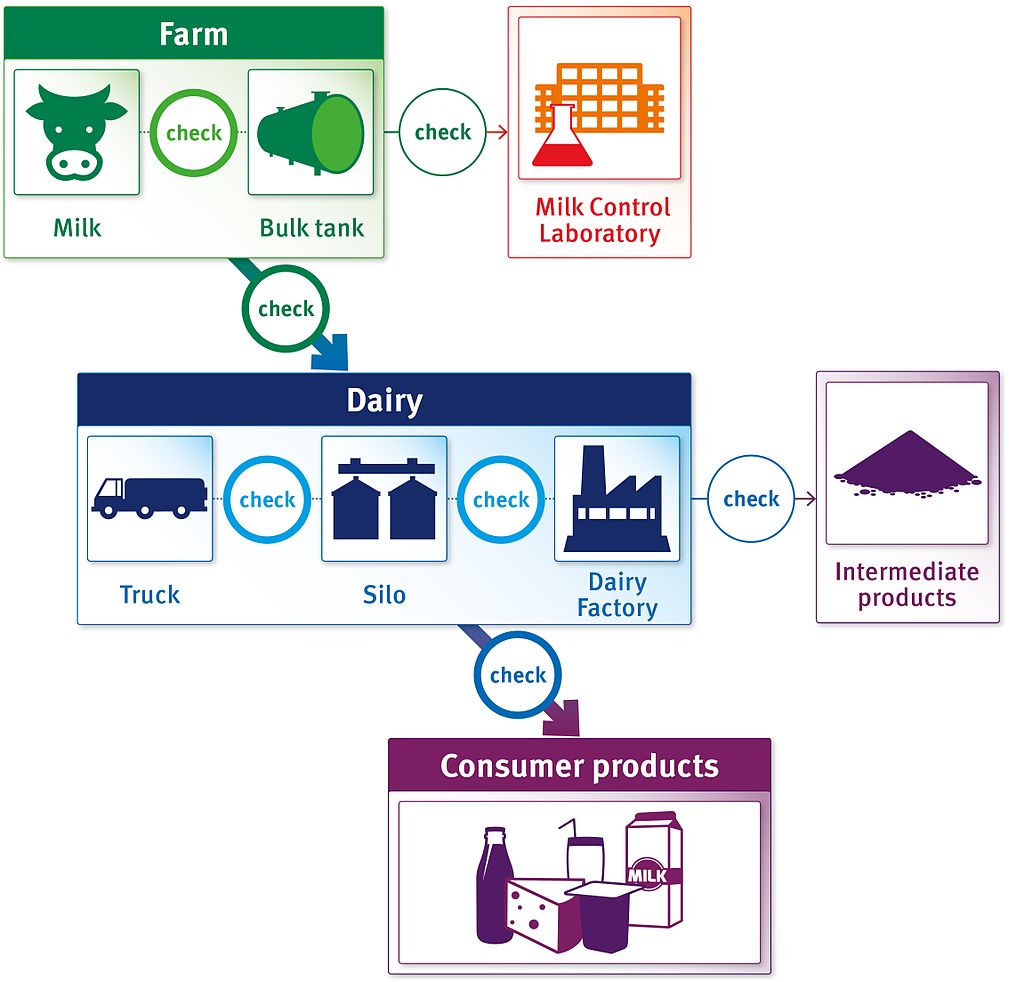
Around the world, countries are changing their antibiotic use with major efforts ongoing to promote and ensure responsible use in both people and in animals.
The FAO/OIE/WHO Tripartite Collaboration on AMR
The WHO, the Food and Agriculture Organization (FAO) and the World Organisation for Animal Health (OIE) speak with one voice and take collective action to minimise the emergence and spread of AMR. The aim is to:
In the UK, the sales of veterinary antibiotics fell by 50% between 2014 and 2018, with sales of the highest priority critically important antibiotics (HP-CIAs) having dropped 66% in this period4. As of 2019, six of the major supermarkets have bans on their suppliers using antibiotics routinely for disease prevention (Co-op, Lidl, M&S, Sainsbury’s, Tesco, and Waitrose). Antimicrobials are necessary to prevent diseases in animals and to look after animal welfare, which is a legal requirement while at the same time making sure we have enough food to feed our population and using antimicrobials appropriately can protect them for future use.