This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 Chemistry
Chemistry
 Science
Science
 Human biology
Human biology
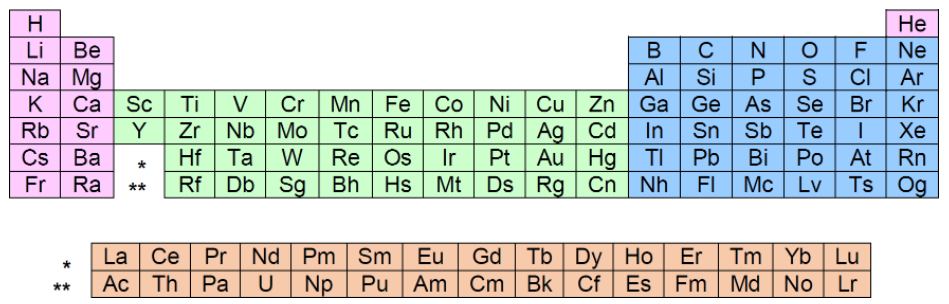
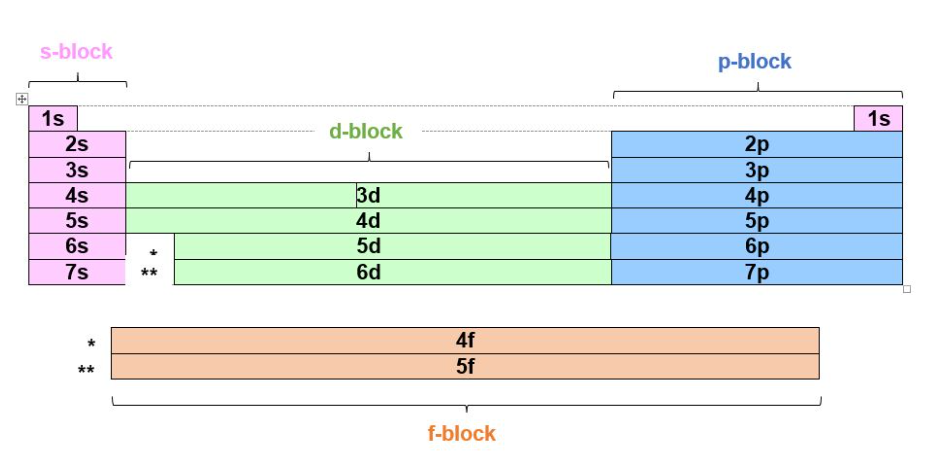
To know which orbitals are found within which electron shells or energy levels we look towards the periodic table:
We can divide the periodic table into sections, which will help tell us where to place electrons.
To determine electronic configuration we must remember:
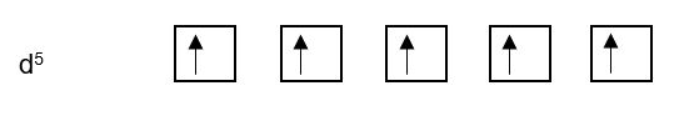
Hund’s rule of maximum multiplicity: For a given electronic configuration with one or more open electronic shells (ie. one or more valence orbitals which are not completely filled with electrons), the lowest energy term is the one with the greatest spin multiplicity.
This sounds complicated, but essentially this means that the most stable (lowest energy) electronic configuration is one where electrons are placed in separate degenerate orbitals prior to being paired. This is commonly referred to as “spin aligned” as opposed to “spin paired”.
Remember: There are 3 degenerate p-orbitals, 5 degenerate d-orbitals and 7 degenerate f-orbitals. Each orbital can hold up to 2 electrons.
Example: