This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
 Biology
Biology
 PSHE / Citizenship studies
PSHE / Citizenship studies
 Science (applied)
Science (applied)
Insulin is a fairly recent discovery: it was first extracted from a dog in 1921 by Banting and Best. In 1922 the same scientists carried out the first insulin treatment by injecting it into a diabetic boy, thereby saving his life. For the next 60 years, people with diabetes were treated by injecting purified pig or pig-and-cow insulin. As the insulin molecule of each species is slightly different, humans can have allergic reactions to these therapeutic insulins; in addition, some animal viruses and other diseases may be passed to patients as the purification process isn’t completely effective.
Recombinant insulin treatments were developed through the 60s and 70s, and in 1982 the first recombinant human insulin was approved for pharmaceutical use. Despite the amino acid sequence (shown below) of this therapeutic insulin being exactly the same as that found in humans, it was still assumed to be a completely new molecule when its safety was reviewed by government officials. Luckily, insulin is a fairly small and simple protein, and therefore can be easily produced by bacteria: some therapeutic proteins, such as blood clotting factors, are too complex to be made correctly by prokaryotic cells and therefore have to be produced by transgenic plants and animals which are much more complicated to genetically engineer.
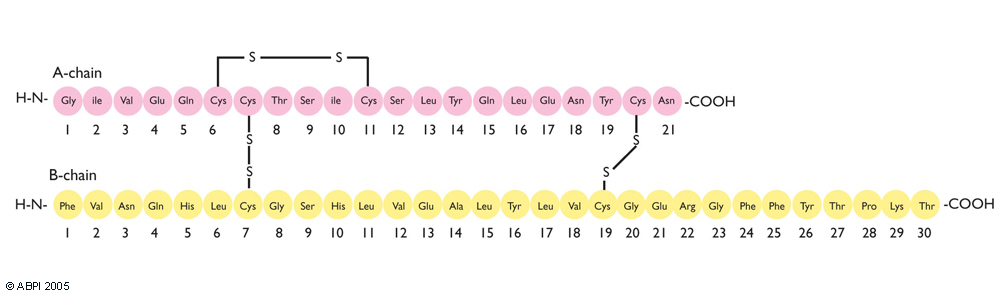
The two chains of human insulin are made by two different strains of genetically modified bacteria.
The insulin is produced by bacteria: for safety, a special strain of E. coli is used that cannot survive outside the lab. Should it somehow escape and mutate so it is able to survive and then infect other organisms, their health won’t be affected by unstoppable insulin production. The A and B chains of insulin are produced separately by different bacteria and then combined to form the therapeutic hormone once they’ve been purified.
The genetic engineering process uses a plasmid, as shown in the animation below.
Now that recombinant human insulin is a common treatment, scientists are improving its action by changing the DNA sequence before it is introduced into the bacteria so that a slightly different protein is produced. The original therapeutic human insulin had to be taken one or two hours before eating (its circulation around the body is slightly different when it is injected compared to when it is produced by the pancreas), but this meant patients didn’t always take their insulin at the correct time.
Insulin analogues have been developed that act faster so they can be taken just before a meal like the short-acting insulin in the animation below; other long-lived analogues have also been developed so insulin only has to be injected once a day. Unfortunately, insulin is not a stable molecule (changes in its structure occur during handling, storage and even during use) so changes to its DNA sequence and therefore protein structure have been made that improve its stability and therefore the efficiency of treatment.
Whilst insulin is the recombinant hormone used to treat the largest number of people, other hormones are also badly needed. Human growth hormone (HGH) is a protein hormone produced in the pituitary gland which is needed for children to grow. Children who do not make enough HGH fail to grow normally. Unlike insulin, animal growth hormone has no effect in humans, so in the past the hormone needed to treat affected children had to be extracted from the pituitary glands of dead bodies. Unfortunately, there was not enough HGH available to treat all those who needed it; even worse, Creutzfeldt-Jakob disease (sometimes referred to as ‘mad cow disease’) could be transmitted to the patient through HGH from an infected pituitary gland donor. Scientists have genetically modified E. coli, adding the genes needed to make functional human HGH. As a result, there is a plentiful, regular, disease-free supply of the hormone for those children who need it to grow normally. This is another successful use of genetic modification to produce a valuable therapeutic compound.